Obstructive vs Non-Obstructive Azoospermia: Causes and Treatments
Azoospermia means there is no sperm found in the ejaculate on properly performed semen testing. It affects roughly 1 in 100 men and around 10–15% of men evaluated for infertility. Understanding why no sperm is present is crucial, because the treatment path differs completely depending on the cause.
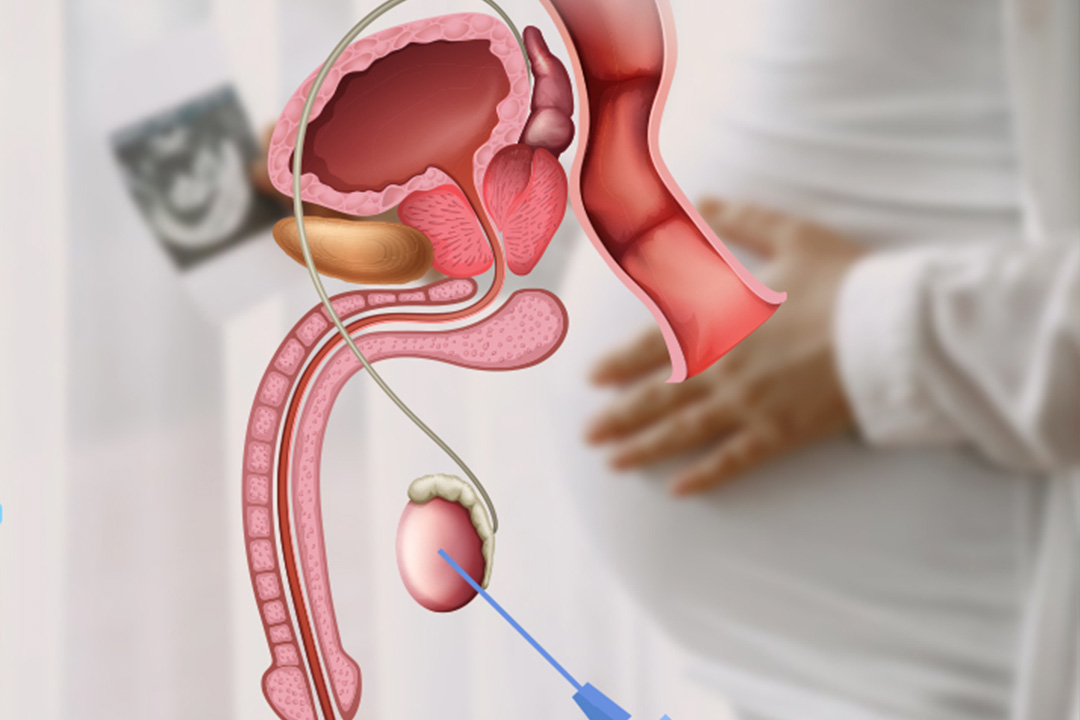
In obstructive azoospermia, the testes are making sperm, but a physical blockage or disconnection prevents sperm from reaching the semen. In non-obstructive azoospermia, the issue lies in sperm production itself (spermatogenesis), often due to genetic factors, hormonal problems, prior illness, or testicular damage.
Correct diagnosis requires a structured evaluation like history, examination, repeat semen testing with centrifugation, selective blood tests, and targeted imaging. It can open doors to surgical repair, medical therapy, or sperm retrieval with assisted reproduction.
Obstructive vs Non-obstructive Azoospermia
| Feature | Obstructive Azoospermia | Non-Obstructive Azoospermia |
|---|
| Core problem | Blockage/absence in the sperm transport pathway after sperm is made (epididymis, vas deferens, ejaculatory ducts) | Sperm production in the testes is severely reduced or absent |
| Typical exam & labs | Testes often normal in size/feel; follicle-stimulating hormone (FSH) often normal; vas deferens may be absent in some cases | Smaller, softer testes are more common; FSH often elevated |
| Semen clues | Normal volume and pH in many; low volume + acidic pH suggests ejaculatory duct obstruction | Volume/pH usually unhelpful for diagnosis |
| Common causes | Prior vasectomy or injury, infections/scarring, congenital bilateral absence of the vas deferens (CFTR-related), ejaculatory duct cysts/obstruction | Genetic (karyotype abnormalities, Y-chromosome microdeletions), prior mumps orchitis, undescended testes, chemo/radiation, idiopathic testicular failure, hormonal (e.g., low pituitary signals) |
| First-line management | Microsurgical reconstruction (vasovasostomy/vasoepididymostomy) where suitable; transurethral resection for ejaculatory duct obstruction; or sperm retrieval from epididymis/testis with ICSI | Treat correctable endocrine causes; micro-TESE to retrieve sperm for ICSI if production is patchy; counseling when retrieval isn’t possible |
| Genetic testing | CFTR testing when the vas deferens is absent or obstruction is suspected | Karyotype and Y-chromosome microdeletion testing are standard; additional tests based on history |
How Do Doctors Tell Which Type of Azoospermia it is?
They combine repeat, properly processed semen analysis with a focused exam, hormone tests, and only when clues point to a blockage, targeted imaging.
Azoospermia is confirmed after centrifuging the sample and checking the pellet; laboratories should repeat testing at a separate visit to avoid false calls. The initial clinical split uses classic markers: semen volume and pH, testis size/consistency, and FSH.
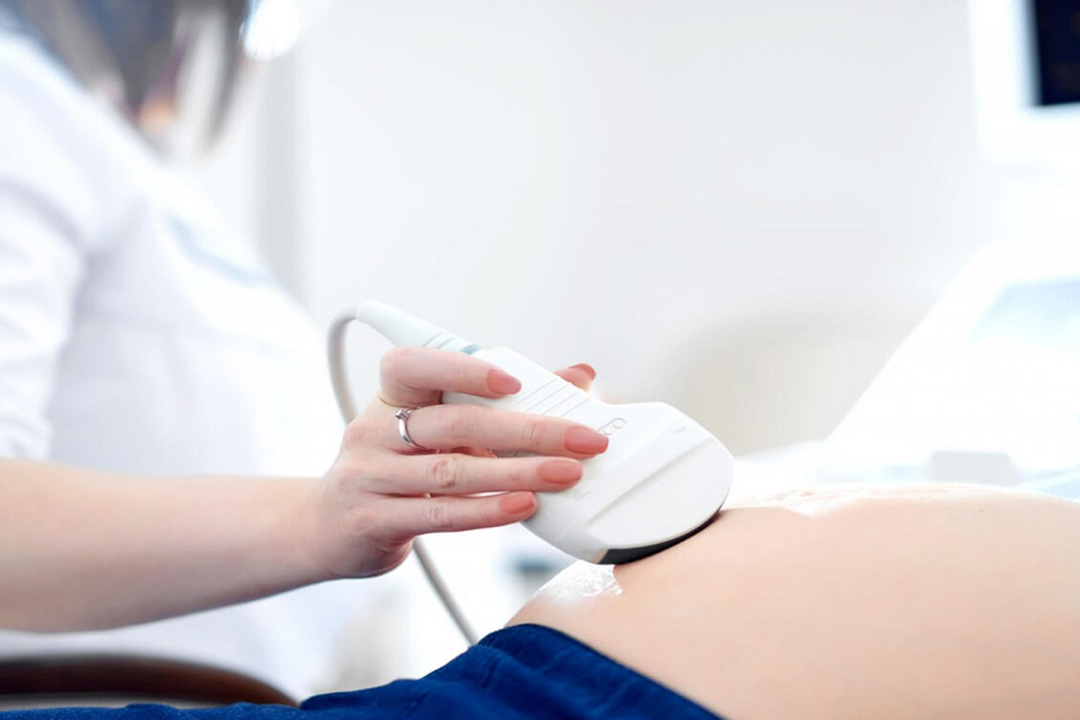
Low volume (<1.4 mL) with acidic pH and normal testosterone plus palpable vas deferens suggests ejaculatory duct obstruction and may justify a transrectal ultrasound to look for dilated seminal vesicles or midline cysts. Otherwise, routine scrotal or pelvic imaging isn’t needed up front.
Causes of Obstructive Azoospermia and Treatment Options
Common causes are prior vasectomy or trauma, scarring from infection, congenital absence of the vas deferens (often CFTR-related), and ejaculatory duct obstruction. Each has a specific fix.
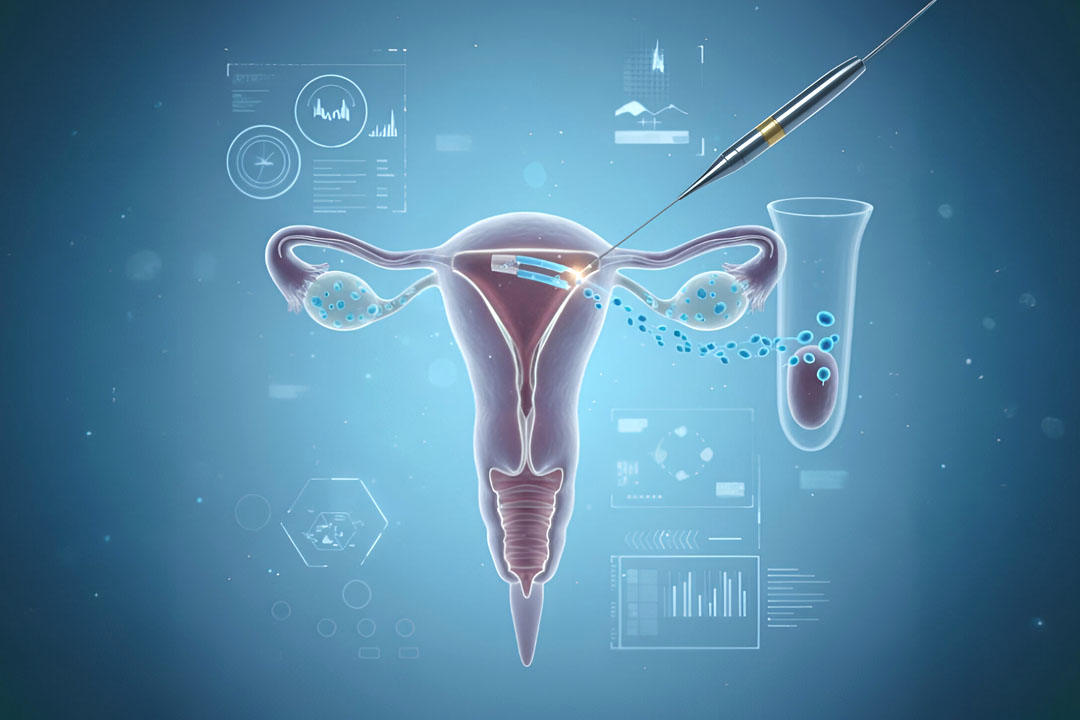
- Vasal/epididymal blockage (including after vasectomy): microsurgical vasovasostomy or vasoepididymostomy can restore sperm to the ejaculate in suitable cases; couples can also choose surgical sperm retrieval with intracytoplasmic sperm injection (ICSI).
- Ejaculatory duct obstruction: if semen is low-volume and acidic and imaging shows features such as dilated seminal vesicles/ducts, transurethral resection of the ejaculatory ducts (TURED) and/or sperm extraction are options.
- Congenital bilateral absence of the vas deferens (CBAVD): this is commonly linked to CFTR variants. Testing the male (and, if positive or absent vas is found, offering genetic evaluation for the female partner) is recommended before treatment choices.
Causes of Non-Obstructive Azoospermia and Treatment Options
Many cases are due to genetic or primary testicular causes; a smaller share stems from hormonal signaling problems that are often treatable.
- Genetic contributors include chromosomal conditions (e.g., Klinefelter) and Y-chromosome microdeletions; guideline panels recommend karyotype and Y-deletion testing in men with azoospermia or very low counts (with strong emphasis when counts are ≤1 million/mL, and consideration when <5 million/mL). These results help with counseling and predict chances of finding sperm at surgery.
- Endocrine (pre-testicular) causes such as hypogonadotropic hypogonadism can often be treated with gonadotropins, potentially restoring sperm production. Hormone manipulation for other non-obstructive cases has limited supporting evidence. Avoid exogenous testosterone when fertility is desired.
- Chemo/radiation history and select infections or undescended testes may lead to lasting testicular damage; in these settings, the main route to a genetic child is sperm retrieval from residual pockets of spermatogenesis plus ICSI.
Evaluation Step-by-Step (What to Expect)
The following steps are involved in the evaluation which type of azoospermia it is, they are:
- History & examination: prior surgeries (vasectomy, hernia), infections, fevers/mumps, medications/supplements, chemo/radiation, sexual function; exam looks for vas deferens, varicocele, testis size/consistency.
- Repeat semen analysis with centrifugation: confirms azoospermia and checks volume and pH.
- Hormones: FSH and testosterone at minimum; further pituitary hormones if low testosterone is confirmed.
- Genetic tests: karyotype and Y-microdeletion for azoospermia or very low counts; CFTR testing if the vas deferens are absent or obstruction is suspected, with partner evaluation as appropriate.
- Targeted imaging: transrectal ultrasound only when semen clues (very low volume + acidic pH) suggest ejaculatory duct obstruction; scrotal ultrasound is not routine for the initial split between obstructive and non-obstructive forms.
Treatment Options for Obstructive and Non-Obstructive Azoospermia
If obstructive azoospermia is confirmed:
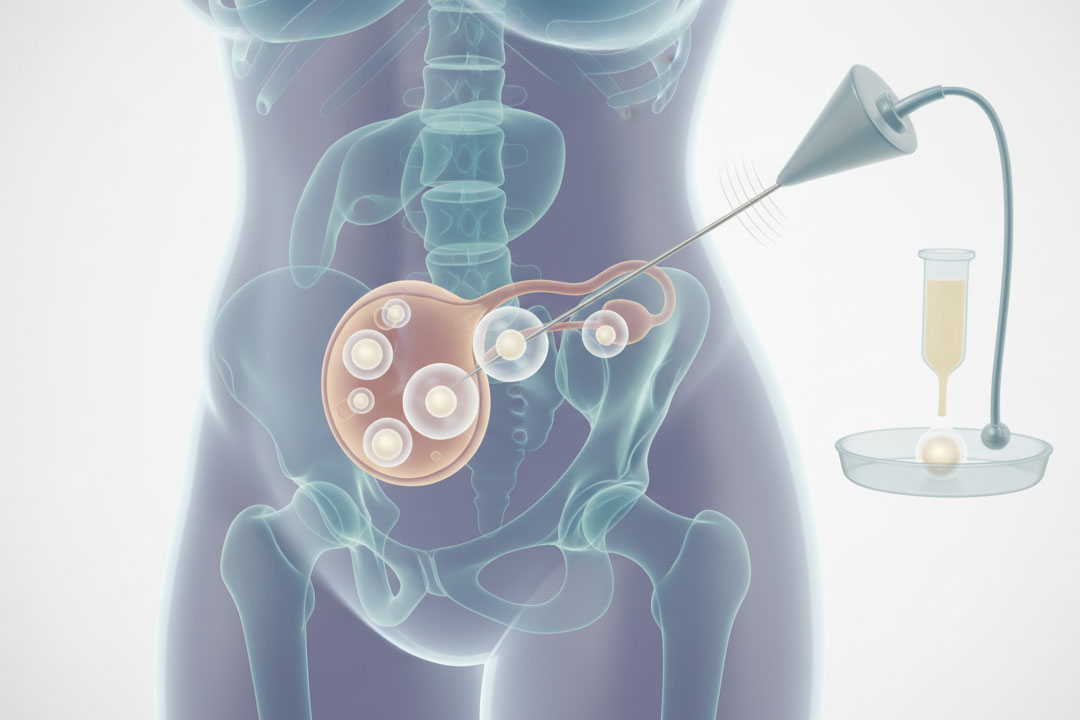
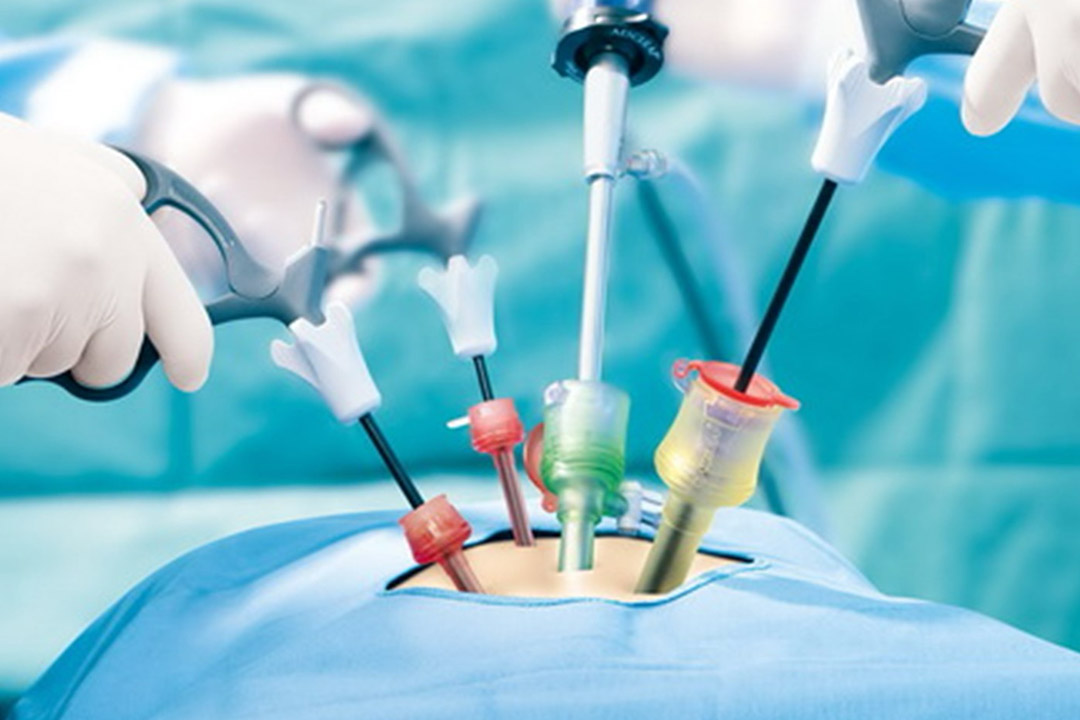
Microsurgical repair (vasovasostomy or vasoepididymostomy) may restore sperm to the ejaculate. When repair isn’t feasible or preferred, sperm retrieval from the epididymis or testis (e.g., PESA/MESA/TESA) followed by ICSI is effective for many couples. Ejaculatory duct obstruction can be treated with TURED in selected cases.
If non-obstructive azoospermia is confirmed:
Treat correctable hormones when present; otherwise, discuss micro-TESE for sperm retrieval with ICSI. Patients should be counseled on expected retrieval probabilities, potential need for multiple attempts, and alternatives (donor sperm, adoption).
Frequently Asked Questions
I am 30, is IVF for me if I have obstructive azoospermia?
If obstructive azoospermia is present and repair is feasible, many couples try reconstruction first; if not, IVF with ICSI using surgically retrieved sperm is a standard path and age 30 itself isn’t a barrier. Success depends mainly on egg quality (partner’s age/ovarian reserve) and whether good-quality sperm are obtained.
Can lifestyle changes reverse non-obstructive azoospermia?
Healthy habits support general reproductive health, but when sperm production is profoundly impaired, lifestyle alone rarely restores sperm in the ejaculate. That’s why the evaluation looks for specific, treatable causes (e.g., hormonal signaling problems) and then considers micro-TESE for retrieval where appropriate.
Do I need genetic testing?
Yes, guidelines recommend karyotype and Y-chromosome microdeletion testing in men with azoospermia or very low sperm counts, and CFTR testing if the vas deferens are absent or obstruction is suspected. Results guide expectations, safety, and family planning.
What are the chances of sperm retrieval with non-obstructive azoospermia?
Across experienced programs, micro-TESE retrieves sperm in roughly 30–60% of men, but this varies by cause and histology. Some genetic patterns (e.g., complete AZFa/AZFb deletions) predict near-zero chance and should be counseled accordingly.
Is micro-TESE safe? Will it affect testosterone?
Micro-TESE is designed to minimize tissue loss compared with non-microsurgical techniques, but temporary or lasting drops in testosterone can still occur; follow-up testing is standard.
If semen volume is very low, does that always mean a blockage?
No, but very low volume with acidic pH and other features can point strongly to ejaculatory duct obstruction, where targeted imaging (transrectal ultrasound) is helpful.
Can medications boost sperm in non-obstructive azoospermia?
When the problem is hypogonadotropic hypogonadism, specific hormone therapy can restart sperm production. Outside of this, evidence for empiric medications is limited, and testosterone therapy should be avoided when trying to conceive.
What if prior cancer treatment caused the problem?
Sperm banking before treatment is best. If azoospermia persists afterward, micro-TESE can still recover sperm in a meaningful minority of men, and ICSI can achieve pregnancies in those cases.
Conclusion
“Zero sperm” is a starting point, not the end of the story. Obstructive azoospermia often has a fix which is to repair the blockage or retrieve sperm and proceed with ICSI.
Non-obstructive azoospermia requires a careful search for treatable hormonal or genetic causes, realistic counseling about micro-TESE retrieval chances, and thoughtful planning for family building.
With guideline-based testing and individualized care, many couples can still pursue a successful path to parenthood.
About Us
AKsigen IVF is a premier center for advanced fertility treatments, with renowned fertility experts on our team. Specializing in IVF, ICSI, egg freezing, and other cutting-edge reproductive technologies, AKsigen IVF is committed to helping couples achieve their dream of parenthood. With personalized care and a patient-first approach, AKsigen IVF provides comprehensive fertility solutions under one roof.