Future of IVF in India: Trends, Innovations and Challenges
India’s fertility landscape is changing fast. People are marrying later, career paths are longer, and medical conditions that affect fertility are being diagnosed more often and earlier. Globally, infertility is common as about one in six adults will experience it at some point in their lives.
Over the past two decades, IVF has moved from niche to mainstream, helped by better labs, safer drugs, and clearer rules. At the same time, technology is reshaping care: digital health for access, gentler stimulation for safety, and data-driven tools to support decisions. New laws now define who can offer treatment and how, while the data-privacy regime is maturing.
Put together, these forces point to a future where IVF in India becomes more regulated, more equitable, more personalized and steadily safer.
IVF Demand in India
Delayed childbearing, high lifetime infertility rates, and wider social acceptance will keep demand strong. Later marriage and parenthood shift more pregnancies into the mid-to-late 30s when egg quality naturally declines, lifting IVF need. The World Health Organization’s 2023 estimate that roughly 17.5% (about 1 in 6) of adults experience infertility underscores the population-level need for services.
Earlier WHO work put the global burden at ~48.5 million couples, a reminder that demand has been large for years, not just recently. Awareness and acceptance of fertility care have grown as well, including among single women who are eligible for ART under India’s law.
How Do India’s New Rules Shape IVF’s Future?
The Assisted Reproductive Technology (Regulation) Act, 2021 and its Rules (2022) make IVF safer and more standardized by registering clinics, setting donor limits, and prescribing storage/record-keeping. The Act establishes a national registry of clinics/banks, age bands for donors and recipients, consent and insurance requirements for oocyte donors, and storage limits for donor gametes and embryos.
In parallel, the Surrogacy (Regulation) Act, 2021 (with 2024 rule changes) keeps surrogacy altruistic and narrowly eligible (including specific conditions for widows/divorcees and updated allowances around donor gametes). Together, these laws nudge the market away from informal practices, raise baseline quality, and protect patients' key for long-term trust in IVF.
IVF availability in India
ART services have historically clustered in richer, urban regions. A 2024 review of fertility care in lower and middle-income countries (including India) describes urban concentration and access gaps.
Telemedicine helps: India’s Telemedicine Practice Guidelines (2020) created a nationwide framework, and the government’s eSanjeevani platform has delivered hundreds of millions of consultations, showing how remote follow-ups, counseling, and medication reviews can work at scale. Expect IVF consults, pre-cycle optimization, and post-transfer care to increasingly blend in-person visits with secure telehealth.
Innovations in IVF Technology
Various innovative technologies from smarter selection, safer stimulation, better labs, and clearer add-on evidence help to achieve better results. Some examples are:
AI-assisted Decision Tools
Early randomized evidence suggests AI systems that score embryos can perform about as well as embryologists on implantation outcomes, not yet clearly better. The hype will settle into practical use: decision support, quality control, and triage especially in high-volume labs rather than silver bullets. Meanwhile, a large randomized trial showed time-lapse incubators (often bundled with AI) did not improve live-birth rates versus conventional culture; clinics should be guided by evidence, not marketing.
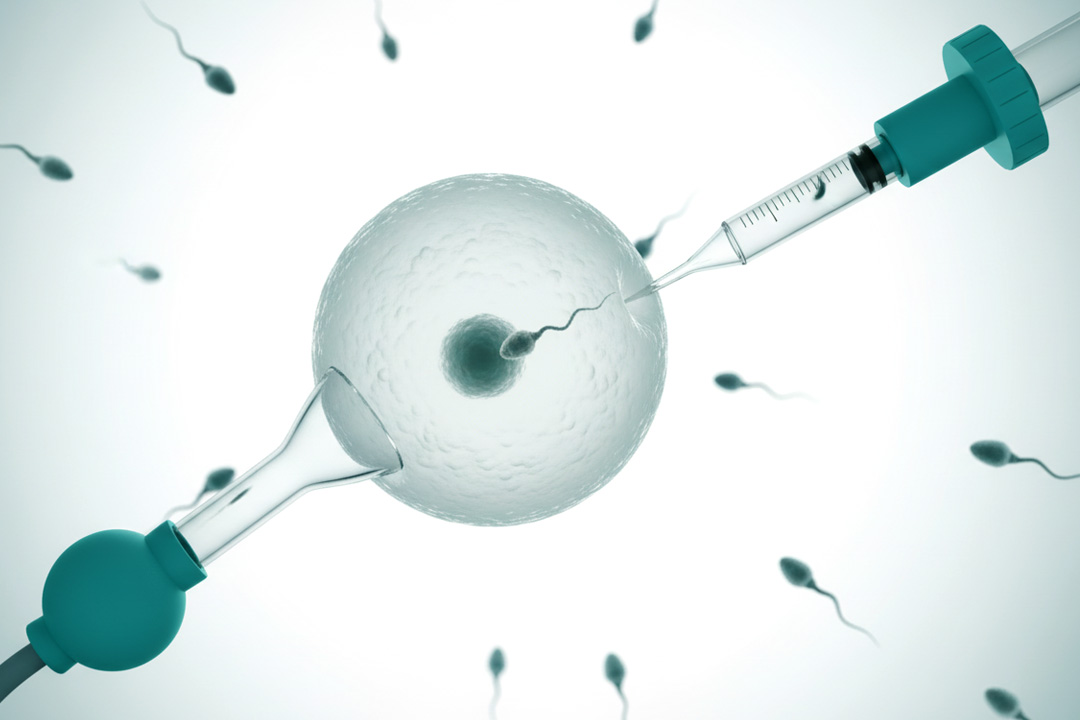
Objective semen analysis
Machine-learning applied to sperm images/motion can standardize morphology and motility assessments, reducing operator variability and potentially improving ICSI sperm selection when evidence matures.
Personalized stimulation and safer triggers
Wider use of antagonist protocols and individualized dosing reduces OHSS risk while maintaining yield; this trend supports a shift toward single-embryo transfer strategies that minimize twins without compromising cumulative success (a global best practice reflected in professional guidance).
Add-ons under scrutiny
Authorities abroad rate add-ons (like PGT-A or ERA) by evidence of strength. The UK regulator’s traffic-light system currently finds no consistent live-birth benefit for several popular add-ons across most patients, though there may be specific subgroups where risk reduction (e.g., miscarriage) is plausible. India’s future will mirror this: more transparent counseling, better consent documents, and restrained use of add-ons unless clear indications exist.
What about Data Privacy as Clinics “Go Digital”?
IVF care generates intimate digital records like photos, videos, lab logs, genetics. The DPDP Act requires consent-based processing, user rights (access/correction/erasure), and penalties for breaches, with implementing rules still rolling out. As AI tools enter labs, compliant data pipelines and audit trails will become essential parts of accreditation and patient trust.
Is Gene Editing Part of IVF’s Near Future in India?
No, germline editing for reproduction is off-limits in India. National guidance allows only somatic gene therapy research in humans; germline modification remains prohibited. So while global headlines appear periodically, Indian clinics will not be offering embryo gene editing for reproduction. Expect research to stay focused on non-invasive embryo assessment, culture media optimization, and safe cryopreservation—areas allowed within current rules.
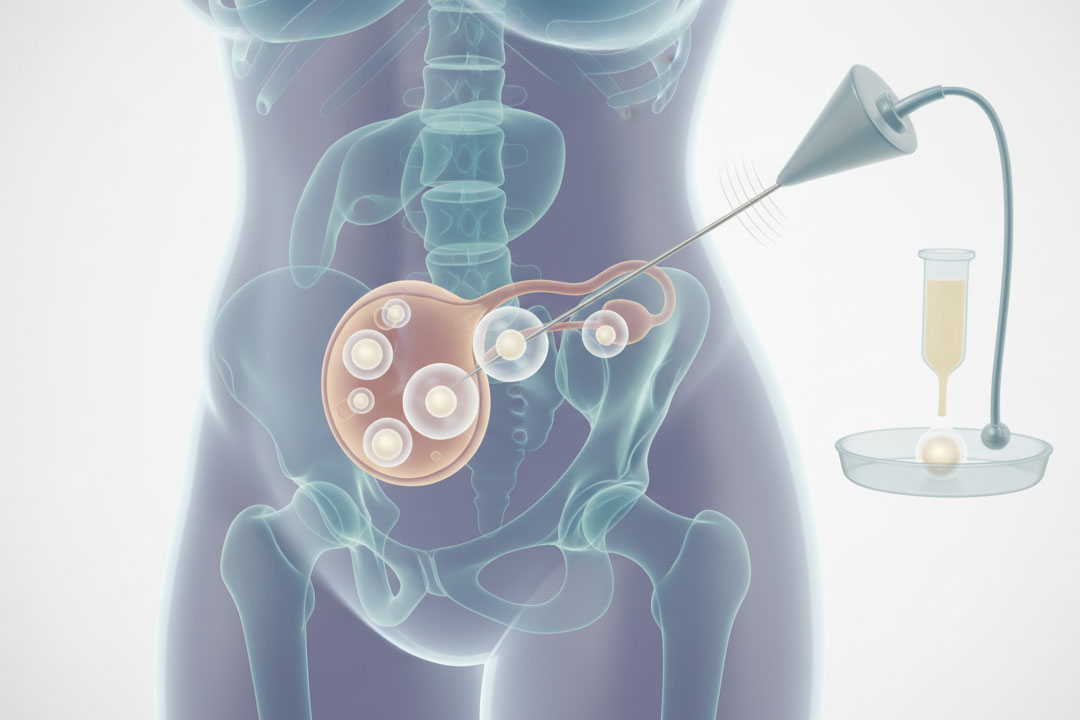
Will freezing eggs, embryos, or ovarian tissue expand?
Yes, fertility preservation will keep growing. Especially elective egg freezing and onco-fertility. India’s ART Rules explicitly allow cryopreservation (with conditions), and global data for ovarian tissue cryopreservation are steadily improving, with growing pregnancy/live-birth reports in selected indications.
Expect wider counseling on preservation options for patients facing gonadotoxic treatments and for those planning pregnancies later in life.
“Natural” or Low-Dose IVF Cycles
Gentler stimulation is likely to grow, but it trades per-cycle success for lower drug exposure and cost so cumulative planning matters. Minimal-stimulation or natural-cycle IVF can reduce side effects and cost per cycle.
However, conventional stimulation still yields more eggs per cycle and usually higher per-transfer success. The future is individualized: using ovarian reserve markers to choose protocols that balance safety, cost, and time-to-baby for each patient.
Near-term Predictions (3–5 years)
Some important changes in IVF treatment across the nation will include:
- Baseline good practice becomes universal: Registered clinics, documented consent, audited labs, and standard counseling on add-ons and multiple-pregnancy risks.
- Blended care models: First opinions, counseling, and many follow-ups by secure telemedicine; in-person for scans, retrievals, and transfers.
- Decision support, not decision replacement: AI tools help embryologists triage embryos and streamline documentation; clinics adopt validation studies before clinical rollout.
- Fertility preservation mainstreams: More elective egg freezing in metros; stronger onco-fertility pathways in tertiary centers; cautious expansion of ovarian tissue work for specific cases.
- Privacy and cybersecurity by design: Clinics update consent forms, vendor contracts, and data retention policies to align with DPDP.
Frequently Asked Questions
Will AI make my IVF “more successful”?
AI can help standardize embryo scoring and lab workflows, but studies so far show non-inferiority to expert embryologists rather than big jumps in live-birth rates. Expect it to complement human expertise, not replace it.
Is time-lapse imaging worth it?
A recent randomized trial found no live-birth advantage over conventional culture. If offered as a paid add-on, ask your clinic to explain expected benefits for your case and whether it changes management.
Are add-ons like PGT-A proven for everyone?
No. Regulators abroad rate add-ons by evidence; many have insufficient or mixed support for routine use. Some subgroups may benefit (e.g., certain miscarriage risks), but decisions should be indication-driven and discussed in counseling.
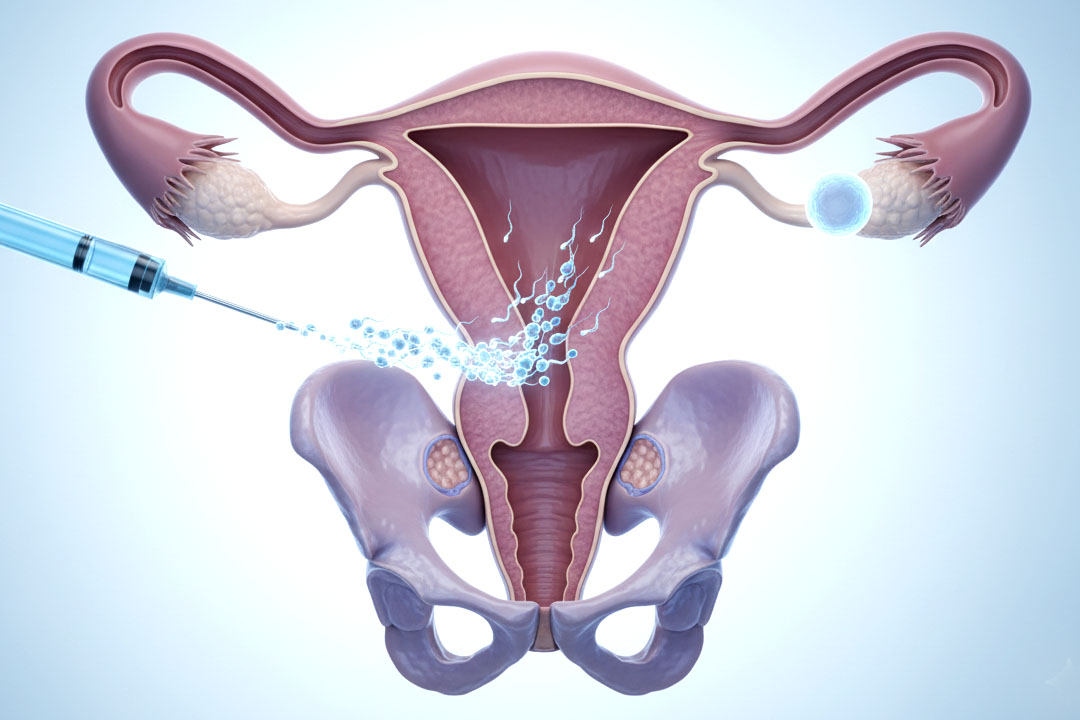
Can single women get IVF in India?
Yes. Under the ART law, any woman aged 21–50 can access ART services; requirements differ for surrogacy, which remains more restricted. artsurrogacy.gov.in
Will clinics keep my data safe if they use apps and AI?
They should. India’s DPDP Act (2023) requires consent-led processing and safeguards for digital personal data. Ask how your clinic secures embryo images, lab videos, and genetic data, and how you can exercise your rights.
Is “natural” IVF better and cheaper?
It can be cheaper per cycle and lighter on medications, but usually retrieves fewer eggs, so you may need more cycles to reach the same chance of a baby. Your ovarian reserve and timeline determine whether it’s a good fit.
Is gene editing part of IVF here?
No. Germline editing is prohibited in India. Reproductive care will focus on safer labs, validated selection tools, and ethically sound research.
Conclusion
India’s IVF future is defined by steady, evidence-first progress. Laws now set a common floor for safety and ethics; telemedicine expands reach; and lab tech from improved stimulation to careful use of AI targets marginal gains that add up.
The era of unchecked “add-ons” is giving way to transparent counseling and outcome audits. For patients, that means clearer choices: the right protocol for your biology, a clinic that shows you its data, and a pathway designed for one outcome one healthy baby at a time.
Facts in this article were cross-checked against WHO publications, Government of India statutes/rules, peer-reviewed journals, and national telemedicine guidelines; specialized tools (e.g., AI embryo scoring, time-lapse incubators, PGT-A) were discussed using contemporary evidence reviews and randomized trials
About Us
AKsigen IVF is a premier center for advanced fertility treatments, with renowned fertility experts on our team. Specializing in IVF, ICSI, egg freezing, and other cutting-edge reproductive technologies, AKsigen IVF is committed to helping couples achieve their dream of parenthood. With personalized care and a patient-first approach, AKsigen IVF provides comprehensive fertility solutions under one roof.